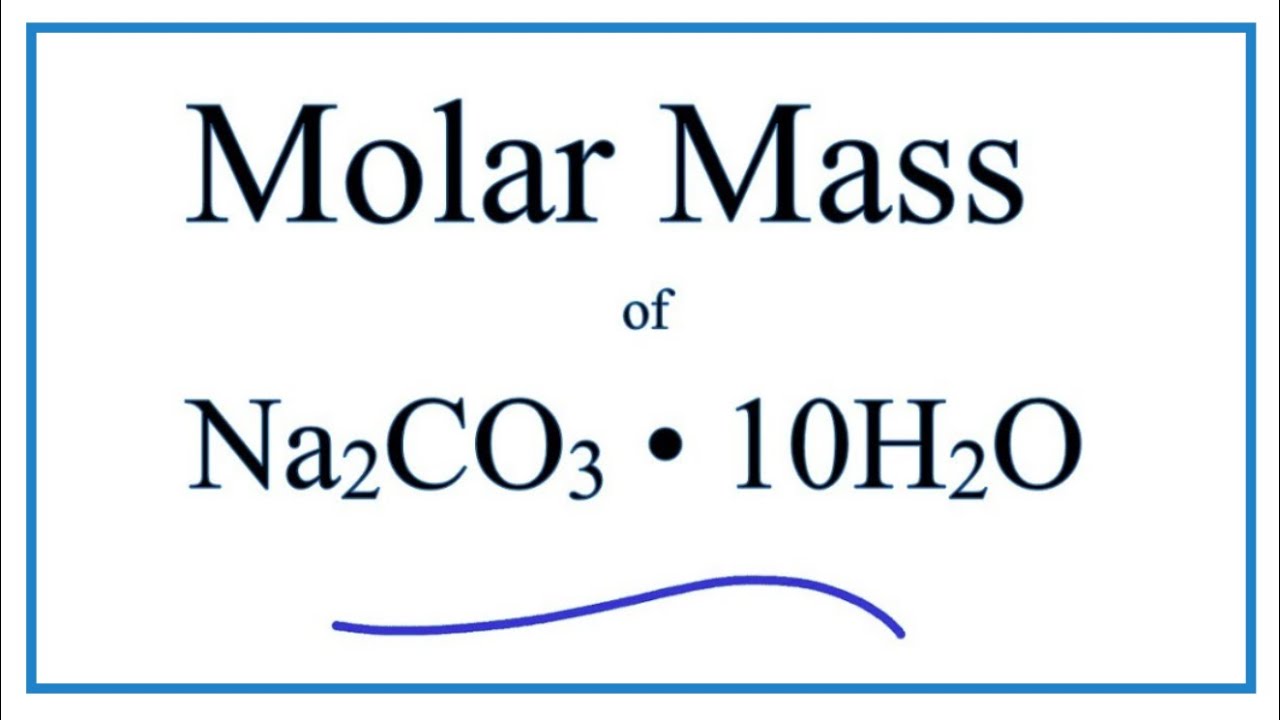
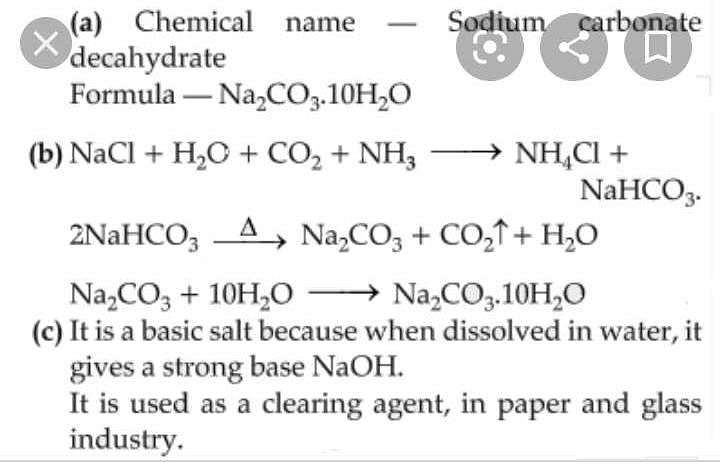
Calculate the formula mass of sodium carbonate (Na2CO3.10H2O) - CBSE Class 9 Science - Learn CBSE Forum

sodium carbonate(Naco3.10H2o)is an important chemical calculate its formula mass in atomic mass unit - Brainly.in

3.12 gm of washing soda crystal (Na2co3.10h2o) were dissolved in 200 mml of water.20 ml of the - Brainly.in
The chemical formula of washing soda is:a)NaHCO3b)Na2CO3.10H2Oc)CaSO4.2H2Od)Correct answer is option 'B'. Can you explain this answer? | EduRev NEET Question

Write the chemical name of Na2 CO3.10H2O and Na2CO3, Write the significance of 10H2OMention the term - Brainly.in

Preparation and thermal properties of Na2CO3·10H2O-Na2HPO4·12H2O eutectic hydrate salt as a novel phase change material for energy storage - ScienceDirect

Construction of Na2CO3·10H2O-Na2HPO4·12H2O eutectic hydrated salt/NiCo2O4-expanded graphite multidimensional phase change material - ScienceDirect

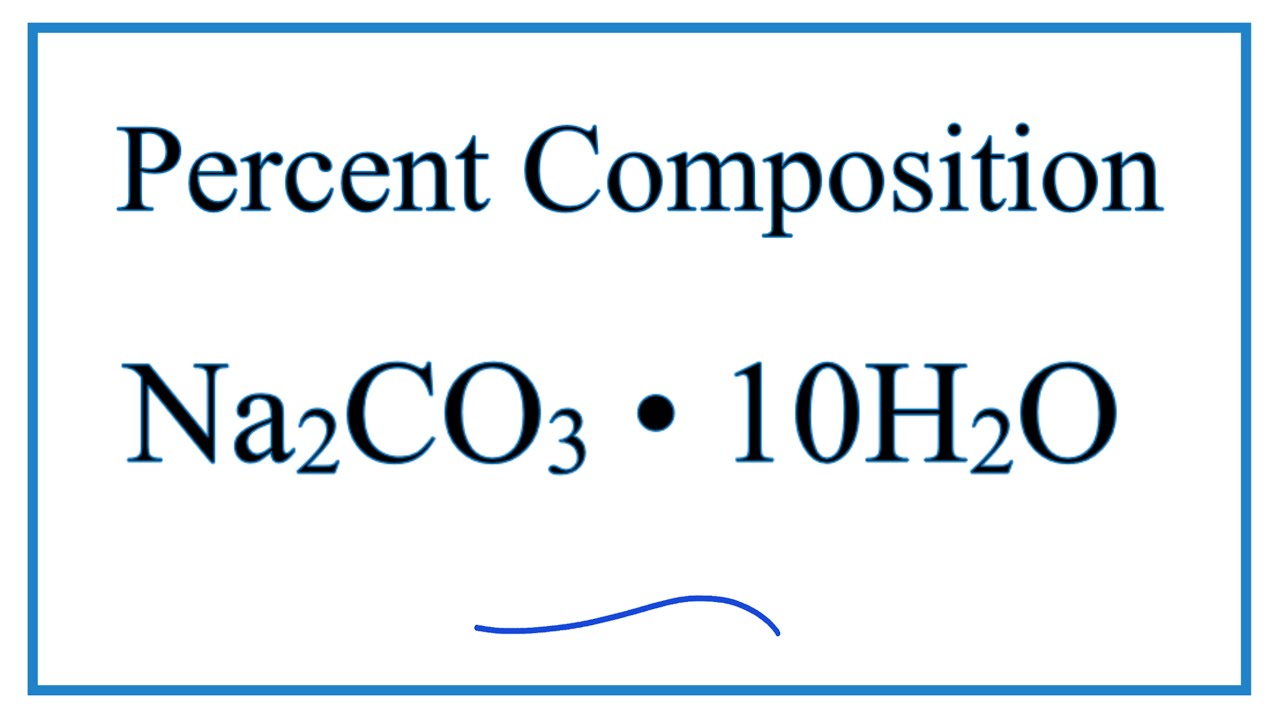
SOLVED: What is the percentage of water in the following compound? Sodium carbonate decahydrate, Na2CO3 • 10H2O % by mass H2O