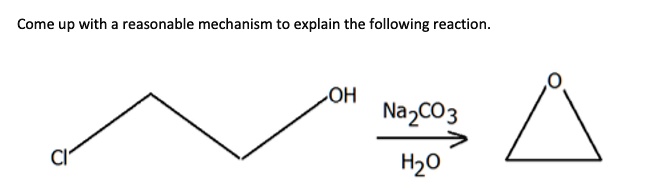
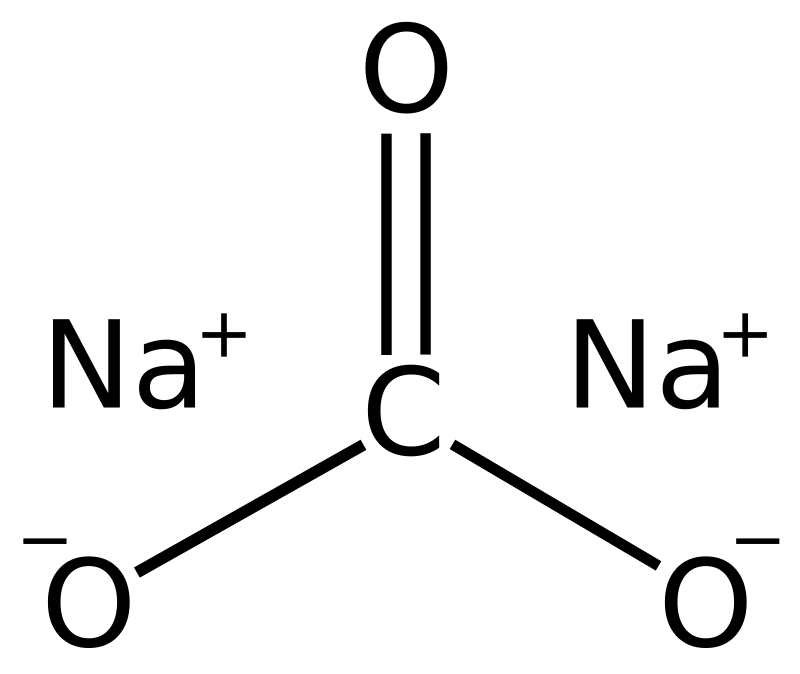
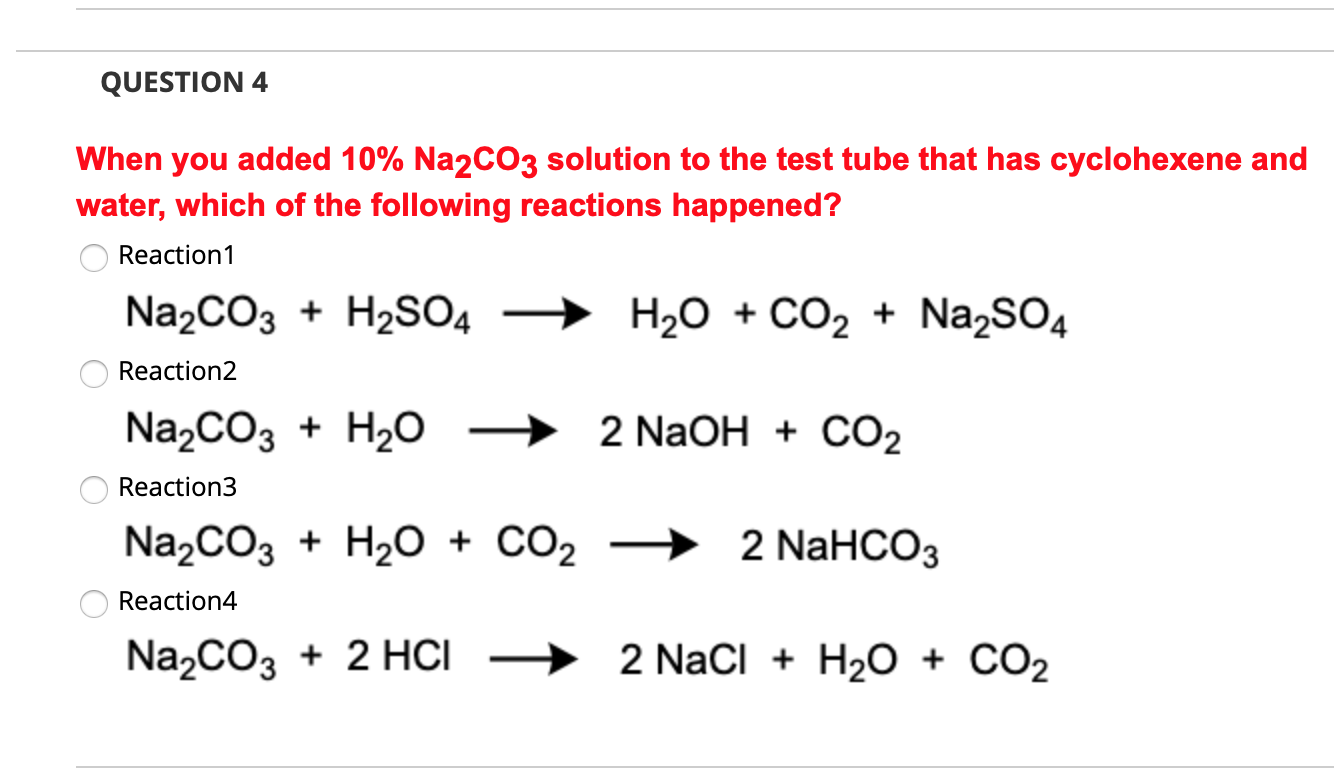
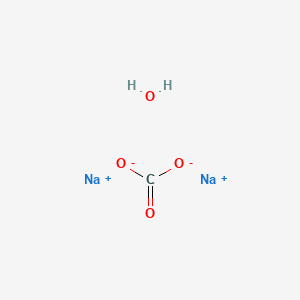
Site-selective Suzuki–Miyaura coupling of heteroaryl halides – understanding the trends for pharmaceutically import

Phase Equilibria of the NaOH–NaBO2–Na2CO3–H2O System at 30 °C, 60 °C, and 100 °C | Journal of Chemical & Engineering Data

Synthesis of compounds 4a–t and 6a–j. Reaction conditions and reagents:... | Download Scientific Diagram