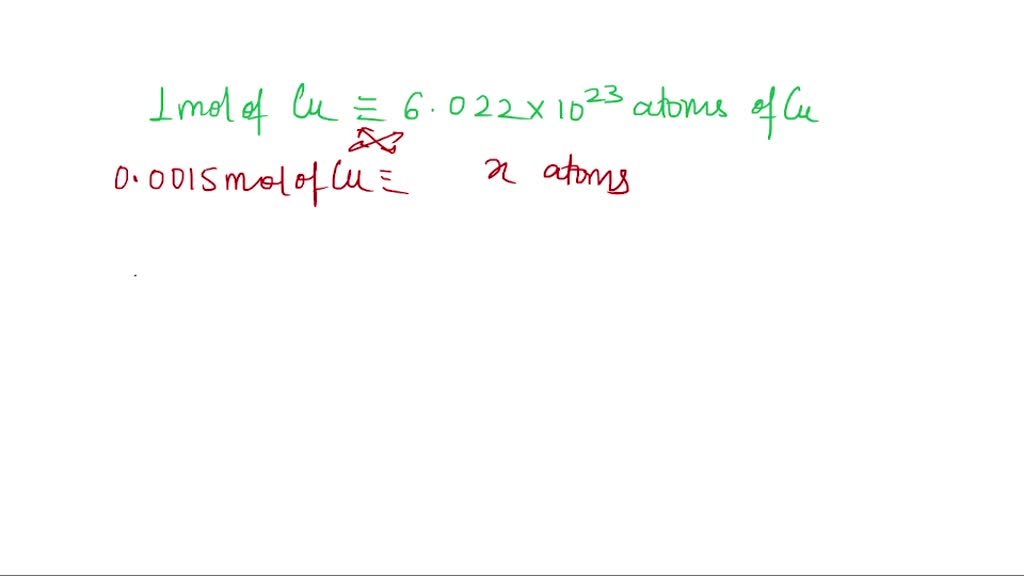Mole Conversions Practice.pptx - Convert 4 moles of Cu(CN)2 to grams 1 mole Cu(CN)2 = 63.5 Cu 24.0 C (12.0 x 2) 28.0 N (14.0 x 2) 115.5 g 4 mole | Course Hero

Molecular orbital energy diagrams for mononuclear copper peroxo (A) and... | Download Scientific Diagram

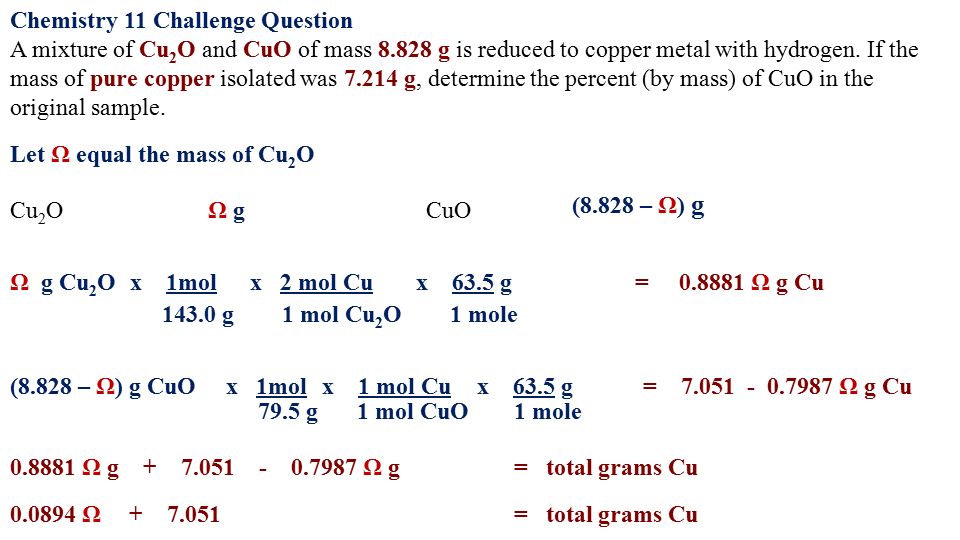
How many grams of Cu would be needed to react with 2.0 mol of HNO3? 3 Cu(s) + 8 HNO3(aq) --> 3 - Brainly.com

Correlating the Experimentally Determined CO Adsorption Enthalpy with the Electrochemical CO Reduction Performance on Cu Surfaces - Xiong - 2023 - Angewandte Chemie International Edition - Wiley Online Library
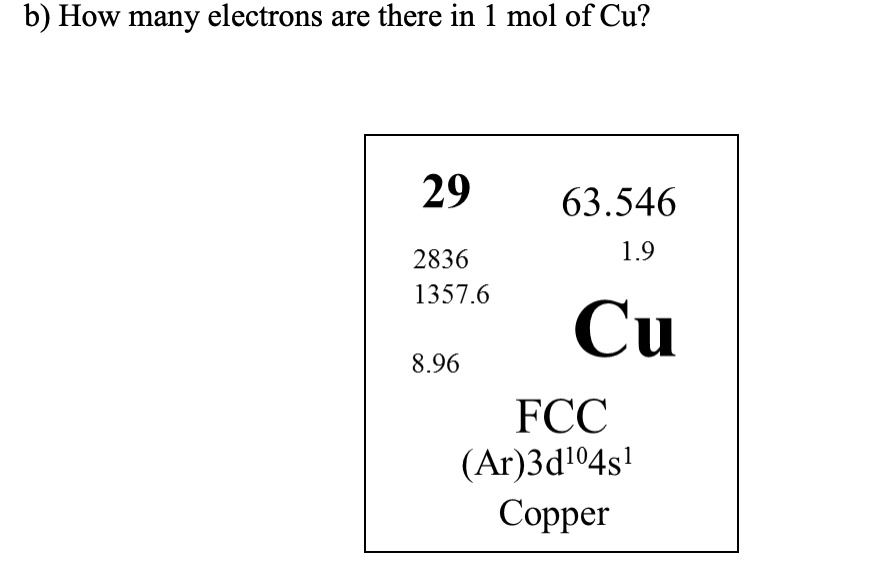
SOLVED: b) How many electrons are there in 1 mol of Cu? 29 63.546 1.9 2836 1357.6 Cu 8.96 FCC (Ar)3dl4s! Copper

SOLVED: What is the volume V of a sample of 2.00 mol of copper? The atomic mass of copper (Cu) is 63.5 g/mol, and the density of copper is 8.92×103kg/m3. express the
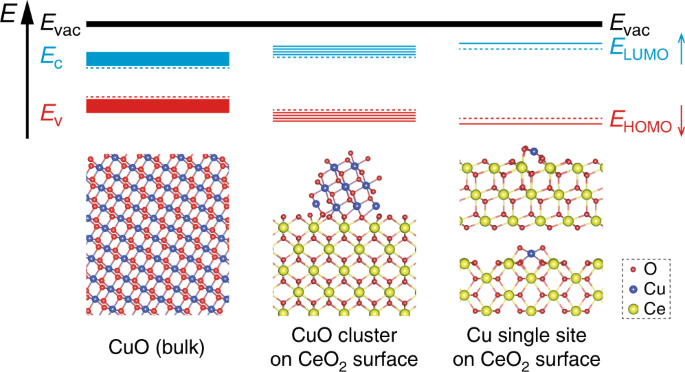
Adsorption and activation of molecular oxygen over atomic copper(I/II) site on ceria | Nature Communications